Introduction

Cesium metal sample in glass.
Quick navigation:
This is part of the chemistry for electronics. Cesium has several practical applications due to its unique properties; here I'm only interested in electrical properties. This also explores work function and bandgap in relation to photocells.
My hybrid approach—electrons moving from negative to positive, with potential energy highest at positive, decreasing to ground—clarifies electron emission in semiconductor devices, as in my xAI debate.
Cesium Properties
Cesium is rare and chemically related to sodium, lithium, and potassium. It is atomic number 55 and atomic weight of 132.91.
Cesium-137 (Cs-137) is radioactive. It is a radioactive isotope of cesium with a half-life of about 30.17 years, decaying mainly by beta emission to barium-137m, which is further followed by gamma emission. This isotope is notable for being produced by nuclear fission and is often associated with nuclear accidents or the operation of nuclear reactors.
Cesium-137 is a radiation hazard due to the body absorbing the isotope and using this to replace potassium.
It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C (83.3 °F; 301.6 K), which makes it one of only five elemental metals that are liquid at or near room temperature. Cesium has physical and chemical properties similar to those of rubidium and potassium. Wiki
Cesium is considered a relatively rare element in the Earth's crust. Due to its low concentration, cesium extraction is complex and often involves by-products from lithium or rubidium mining. The rarity and the difficulty in extracting pure cesium mean it's economically viable only from specific, high-grade sources.
Given these factors, cesium is not as common as elements like silicon, aluminum, or iron but is also not among the rarest elements like astatine or francium. Its rarity, combined with the challenges of extraction, contributes to its value and specialized uses in technology and industry.
Cesium Atomic Clocks

Mini atomic clock module.
Cesium is used as the basis for atomic clocks, but is not radioactive. To quote xAI:
A cesium atomic clock itself is not inherently radioactive in the sense that it does not emit radiation as part of its operation. However, cesium atomic clocks use cesium-133 (Cs-133), which is not radioactive. The clock operates by using the precise microwave frequency at which cesium-133 atoms transition between two hyperfine levels of their ground state, known as the cesium resonance frequency.
Cesium Photoelectric Cells
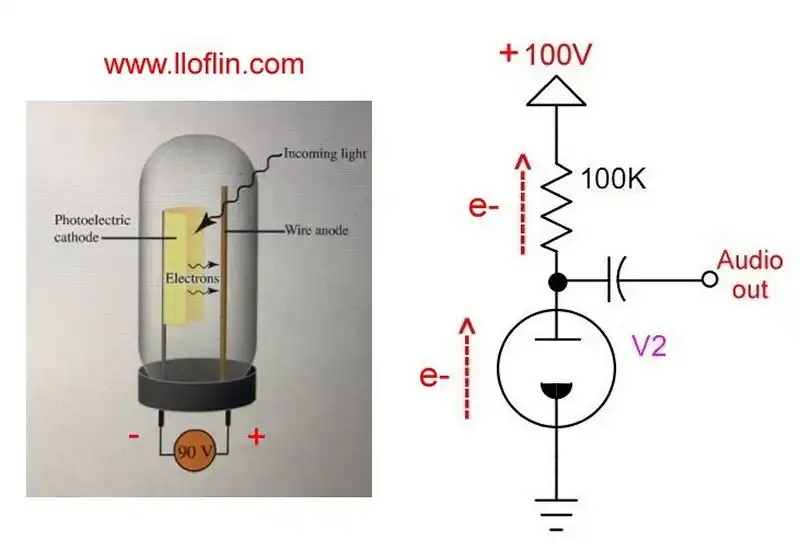
Basic cesium photo vacuum tube operation.
Cesium is used in photoelectric cells due to its low work function, which makes it highly responsive to light. This application is seen in experimental photovoltaic devices and other light-sensitive devices. Due to its ability to emit electrons when heated (thermionic emission), cesium is used in vacuum tubes to improve electron emission.
Cesium vapor lamps emit light at specific wavelengths and are used in spectroscopy and for specialized lighting applications. Cesium's use in these areas leverages its chemical and physical properties like its high reactivity, low ionization energy, and the unique behavior of its isotopes.
A cesium photocell, also known as a photoelectric cell or phototube, operates on the principle of the photoelectric effect, where light energy is used to eject electrons from a material.
Cathode is coated with cesium or a cesium compound, which has a very low work function (the energy required to remove an electron from the surface). This makes it highly sensitive to light. The anode is typically a metal plate or wire, positioned opposite the cathode within an evacuated glass envelope or in a vacuum.
The space between the cathode and anode is either a vacuum or filled with a low-pressure inert gas (e.g., helium or neon) to prevent electron collisions with air molecules.
When photons (light particles) strike the cesium cathode, they transfer their energy to electrons within the material. If the energy of the photon is greater than the work function of cesium, electrons are ejected from the cathode surface. Due to cesium's low work function, even low-energy photons (like those in the visible spectrum) can cause electron emission. This is particularly useful for detecting low-intensity light.
A potential difference (voltage) is applied between the anode (positive) and cathode (negative), creating an electric field. The emitted electrons are attracted to the positively charged anode, moving through the vacuum or gas, creating an electrical current.
The flow of electrons from the cathode to the anode constitutes an electric current, which can be measured. The magnitude of this current is proportional to the intensity of the light hitting the cathode, provided the light's energy exceeds the work function of the cesium.
Photocells like this are used in light meters, automatic door openers, security systems, and in scientific experiments where precise light measurement is necessary. They're also used in older technologies like early television cameras. Cesium photocells are particularly sensitive to longer wavelengths in the visible spectrum, including red light, which is useful for applications where human eye sensitivity (which peaks in the green) is not the primary concern.
The efficiency of cesium photocells is due to cesium's low work function, which allows for a broad spectral response, making them useful for detecting light across a wide range of wavelengths. However, they are somewhat sensitive to environmental conditions like humidity, which can affect their performance over time.
Electrons flow from cathode to anode, driven by my electron flow approach, where potential energy decreases from positive to ground.
Is Zinc Photovoltaic?
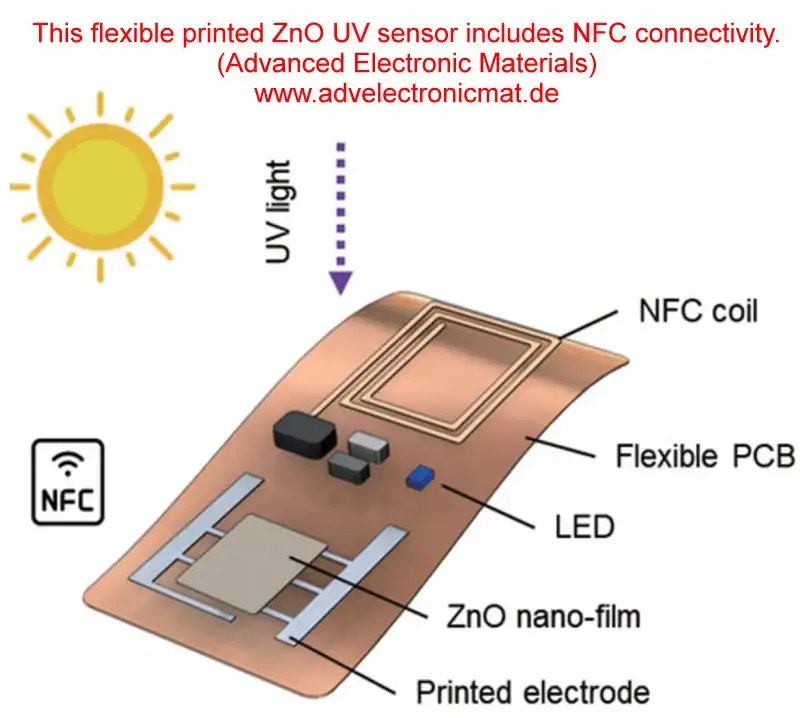
Zinc Oxide UV Detector by Advanced Electronic Materials (advelectronicmat.de).
Zinc oxide (ZnO) is well-known for its applications in UV detection due to its wide bandgap (approximately 3.37 eV), which corresponds to UV light absorption. ZnO can be used to fabricate UV photodetectors because it exhibits high sensitivity to ultraviolet light while being relatively transparent to visible light, making it suitable for "visible-blind" UV detection.
Zinc itself does not produce a voltage in the presence of light like a photovoltaic material would. However, when zinc is used in certain configurations or with other materials, it can be part of a system that generates electricity from light:
Photocatalysis: Zinc oxide (ZnO), for instance, is a semiconductor that can exhibit photocatalytic properties. When ZnO is exposed to light, especially ultraviolet light, it can generate electron-hole pairs. This can lead to a voltage or can be utilized in processes like solar cells or for photocatalytic water splitting, where the light energy is used to generate hydrogen from water.
Dye-Sensitized Solar Cells (DSSCs): Zinc oxide has been used in dye-sensitized solar cells as an alternative to titanium dioxide. In these cells, light is absorbed by a dye, and the excited electrons are transferred to the conduction band of the zinc oxide, which can then be used to generate electrical current.
Galvanic Cells: If you're considering traditional electrochemistry, zinc can be involved in creating a voltage through redox reactions, but this isn't directly due to light. However, in some experimental setups, light can influence the rate or efficiency of these redox reactions by heating or by photo-induced electron transfer, but this isn't zinc producing voltage purely because of light exposure in a direct photovoltaic sense.
Photoelectrochemical Cells: Here, materials including zinc compounds can be used where light influences the electrochemical process. For instance, when zinc compounds are part of an electrode in a photoelectrochemical cell, light can cause excitation of electrons leading to generation of voltage or current.
So, while zinc metal itself doesn't generate a voltage simply by being exposed to light, zinc in various compounds or within specific devices can indeed be part of systems that convert light energy into electrical energy. If you're looking into applications like solar energy conversion, it's the combination of materials and their interactions with light that would be of interest, rather than zinc alone.
Electron-hole pairs in ZnO move under light, aligning with my semiconductor electron flow.
Bandgap vs. Work Function
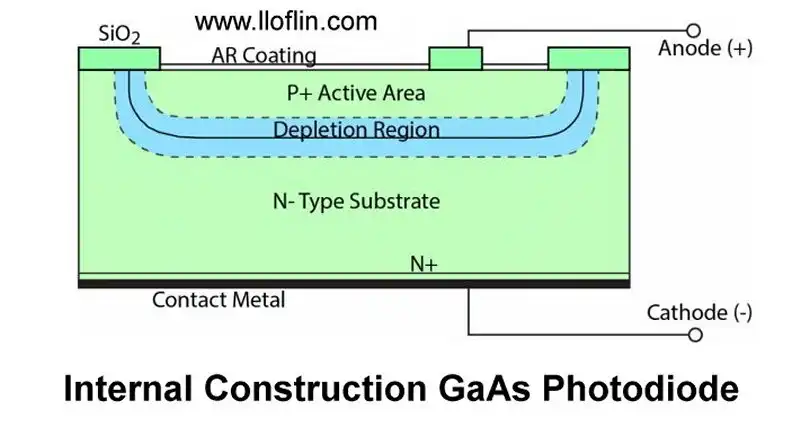
Gallium-arsenide photodiode internal diagram.
A bandgap refers to the energy difference between the valence band and the conduction band in the electronic structure of a material, particularly in semiconductors and insulators.
Valence Band: This is the highest energy band filled with electrons in a solid at absolute zero temperature;
Conduction Band: This is the next band above the valence band where electrons can move freely, thus contributing to electrical conductivity.
Semiconductors: have a bandgap that is relatively small, allowing electrons to jump from the valence band to the conduction band with the addition of thermal energy or light, thereby increasing conductivity. This property is fundamental for the operation of devices like diodes, transistors, and solar cells.
In Insulators the bandgap is very large, meaning it requires a significant amount of energy for electrons to jump to the conduction band. In Conductors there's typically no bandgap, or the bands overlap, allowing electrons to move freely in the conduction band even at low energies.
Bandgap can affect:
Color of light emitted or absorbed by the material (in LEDs or solar cells).
Electrical properties like conductivity, which influences applications in electronics.
Thermal properties since the bandgap determines how easily electrons can be excited by thermal energy.
For a semiconductor like silicon, the bandgap is about 1.1 eV (electron volts), while for gallium arsenide, it might be around 1.43 eV. The exact value can vary slightly with temperature and other conditions.
Yes, both the work function and the bandgap are typically measured in electron volts (eV):
Work Function: This is the minimum energy required to remove an electron from the surface of a material. It's essentially the energy needed to overcome the material's potential barrier at the surface. The work function is indeed measured in electron volts because it represents the energy difference.
Bandgap: This refers to the energy difference between the top of the valence band and the bottom of the conduction band in a semiconductor or insulator. The bandgap determines the energy required to promote an electron from the valence band to the conduction band, where it can contribute to electrical conduction. Like the work function, the bandgap is also measured in electron volts.
Both quantities are fundamental in the study of materials science, particularly in the context of semiconductors, photovoltaics, and electron emission processes. They are crucial for understanding and designing electronic and optoelectronic devices.
Table of common semiconductor bandgaps:
| Material | Bandgap (eV) |
|---|---|
| Silicon | 1.12 |
| Gallium Arsenide | 1.43 |
| Zinc Oxide | 3.37 |
| CIGS | 1.0–1.7 |
Work Function Explained
Work function differs from bandgap.
The work function is a term in physics that quantifies the minimum amount of energy required to remove an electron from the surface of a solid material to a point just outside its surface (into a vacuum). The work function depends on the material's properties, including its crystal structure, surface conditions, and chemical composition. Different materials have different work functions; for example, cesium has one of the lowest work functions at about 2.1 eV, while metals like platinum have higher work functions around 5.65 eV.
In the context of the photoelectric effect, if the energy of an incident photon (given by E = hν, where h is Planck's constant and ν is the frequency of the light) is greater than or equal to the work function of the material, electrons can be ejected from the material.
Work function represents an energy barrier that electrons must overcome to leave the material, impacting how materials respond to light or thermal energy in various applications.
Bandgap and Solar Cells
Bandgap refers to the energy difference between the valence band (where electrons are bound to atoms) and the conduction band (where electrons can move freely and conduct electricity) in a material.
Valence Band - think of this as the "home" where electrons are normally sitting in an atom, not free to move around.
Conduction Band - this is like an "open road" where electrons can move freely, allowing electricity to flow.
The bandgap is essentially the "energy hill" that an electron must climb to jump from its home (valence band) to the open road (conduction band). Here’s what this means for materials. Semiconductors (like silicon or Copper Indium Selenide/Copper Indium Gallium Selenide, known as CIS/CIGS) have a moderate bandgap. When light with enough energy hits these materials, it can give electrons the energy needed to jump this gap, creating free charges that can generate electricity.
Insulators have a very large bandgap, meaning it takes a lot of energy to move electrons to the conduction band, so they don't conduct electricity well. Conductors (like metals) have virtually no bandgap or an overlapping valence and conduction band, so electrons can move freely even without additional energy.
In solar cells, the bandgap is crucial because it determines which wavelengths of light the material can absorb and convert into electrical energy. A smaller bandgap can absorb longer wavelengths (like infrared), while a larger bandgap absorbs shorter wavelengths (like blue or ultraviolet light). The ideal bandgap for solar cells is one that matches well with the solar spectrum to maximize energy conversion from sunlight.
If the bandgap is too small, while the material might absorb more of the solar spectrum, the excess energy from high-energy photons (short wavelengths) is lost as heat, reducing efficiency. If the bandgap is too large, the material might miss out on absorbing lower energy photons, again impacting efficiency.
Thus, for solar cell design, selecting or engineering a material with an optimal bandgap is crucial for maximizing the conversion of solar energy into electrical energy, considering both the energy efficiency and the spectral response of the material.
Conclusion
Cesium and zinc oxide enable light-sensitive devices, driven by electron flow and governed by bandgap and work function. My hybrid approach—electrons from negative to positive—clarifies these, as in my training program. Explore more in my circuit projects or YouTube videos.
Share This Article
References
Paul Sharz, Practical Electronics for Inventors, McGraw-Hill Education.
Stephen L. Herman, Electric Circuits, Delmar Cengage Learning.
Personal correspondence with xAI, 2025.
Lewis Loflin’s teaching materials, Bristol Community College, 1980s–1990s.
Wikipedia, Cesium Properties, accessed 2025.
Advanced Electronic Materials, Zinc Oxide UV Detector Image, advelectronicmat.de.
Related Subjects
- Chemistry for Electronics:
- Why Chemistry is Useful for Learning Electronics
- Electrochemistry and Battery Charger Chemistry
- What is Electrochlorination and Electrolysis?
- Electroplating One Gram of Copper Working Example
- TL431 Battery Charger Voltage Detector Circuits Schematics
- TL431 Sink Mode Constant Current Circuits
- Physics and Chemistry for Electronics:
- Cesium Photo Detectors, Zinc Photocells, and Bandgap Explained
- Cesium Photoelectric Cells
- Is Zinc Photovoltaic?
- Bandgap versus Work Function Key Differences
- How Selenium Rectifiers and Photocells Operate
- Brief Overview of Vacuum Tubes and Circuits
- Basic Review Operation GaAs Photodiodes
- Electronics Education and Careers Defined Six Parts:
- Applied versus Theoretical Science Relation to Electronics
- How does applied science differ from theoretical science?
- Is electronics an applied science?
- What is the difference between electronics and electrical technicians?
- What does "academic ability" really mean in practical terms?
- How does an electronics technician differ from an engineer?
- Electronic Circuits and Applications:
- Electronics and Technology Built at Home Mainpage
- Arduino Constant Current H-Bridge Motor Control
- LM555 Timer Monostable AC Power Phase Control Demo