Introduction to Electrolysis
Chemical process to produce bleach.
Electrolysis is a chemical process driven by passing an electric current through an electrolyte, an ionic substance, either in a solution or molten state.
For example, molten table salt (sodium chloride) becomes conductive, an ionic compound. When an electric current is passed through it, this compound breaks apart into chlorine gas and sodium metal. The process is energy-intensive.
During electrolysis, electrical energy is converted into chemical energy. When an electric current is applied, it forces a non-spontaneous chemical reaction by driving ions to migrate toward oppositely charged electrodes.
I view this through electron flow—electrons move from negative to positive, with potential energy highest at the positive terminal, decreasing to ground. This clarifies processes like battery charging, as discussed in my xAI debate.
Applications of Electrolysis
Applications: Electrolysis has various purposes, including:
Electroplating - to coat one metal onto another.
Electro refining - to purify metals.
Production of chemicals - like chlorine, hydrogen, and sodium hydroxide from brine solution.
Anodizing - to increase corrosion resistance in metals like aluminum.
Electrolysis of water - to produce hydrogen and oxygen gases.
Electrolysis requires an external source of electrical energy because the process is not spontaneous; it's endothermic, meaning it requires energy input to proceed.
The amount of chemical change during electrolysis is proportional to the quantity of electricity passed through the electrolyte.
In summary, electrolysis is a technique where electrical energy is used to drive a chemical change that would not occur naturally by moving ions and forcing them to react at electrodes.
Learn more about electroplating or battery circuits.
Electrochlorination Process

Pool chlorination equipment.
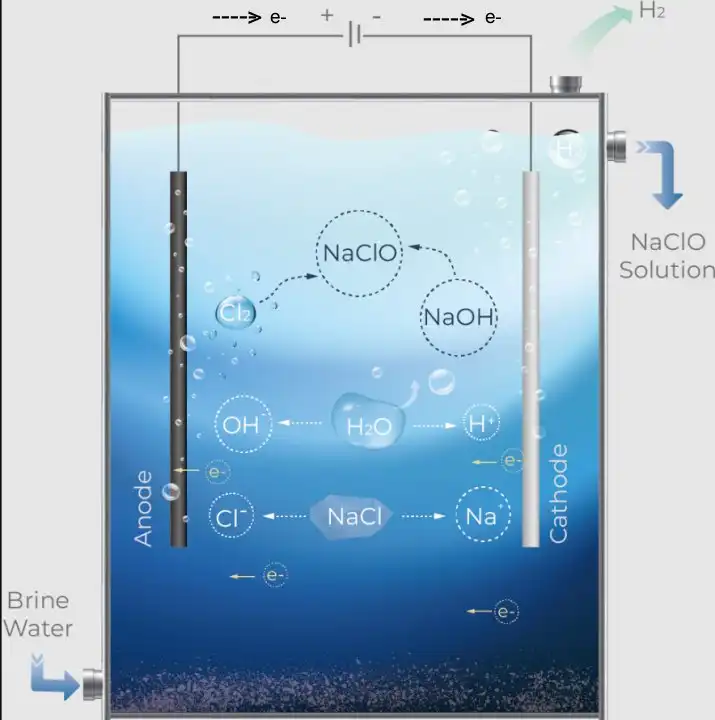
Electrochlorination produces sodium hypochlorite (bleach) by passing an electric current through salt water (brine). This bleach disinfects the water and makes it safe for human use, such as drinking water or swimming pools.
A low-voltage DC is applied, and electrolysis happens, producing sodium hypochlorite and hydrogen gas (H2).
At the negative electrode (cathode), water is reduced (2H₂O + 2e⁻ → H₂ + 2OH⁻), and sodium ions (Na⁺) combine with OH⁻ to form sodium hydroxide (NaOH) and hydrogen gas.
At the positive electrode (anode), chlorine ions (anions) oxidize (lose electrons), becoming chlorine gas (Cl2).
It is the electrolysis of salt water to produce a chlorinated solution. The chlorine gas attacks the sodium hydroxide, producing sodium hypochlorite.
Electrons flow from the cathode to the anode through the external circuit, driving these reactions, as explained in my electron flow model.
Electrochlorination Mechanism

Chlorination electrolysis cell.
The first step is removing any solids from the saltwater. Next, the saltwater streams through an electrolyzer cell's channel of decreasing thickness. One side of the channel is a cathode, and the other is an anode.
The solution travels to a tank that separates the hydrogen gas based on its low density. The simplified chemical reaction is:
NaCl + H2O + energy → NaOCl + H2
That is, energy is added to sodium chloride (table salt) in water, producing sodium hypochlorite and hydrogen gas.
Because the reaction takes place in an unpartitioned cell and NaOH is present in the same solution as the Cl2:
2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2 - any Cl2 disproportionates to hypochlorite and chloride.
Cl2 + 2 NaOH → NaCl + NaOCl + H2O resulting in a hypochlorite solution.
Conclusion
Electrolysis and electrochlorination are vital for electronics and industry, from battery chemistry to producing bleach for safe water. My electron flow approach—electrons driving reactions from negative to positive—clarifies these processes, as seen in my training program. Explore more in my circuit projects or YouTube videos.
Share This Article
References
Paul Sharz, Practical Electronics for Inventors, McGraw-Hill Education.
Stephen L. Herman, Electric Circuits, Delmar Cengage Learning.
Personal correspondence with xAI, 2025.
Lewis Loflin’s teaching materials, Bristol Community College, 1980s–1990s.
Related Subjects
- Chemistry for Electronics:
- Why Chemistry is Useful for Learning Electronics
- Electrochemistry and Battery Charger Chemistry
- What is Electrochlorination and Electrolysis?
- Electroplating One Gram of Copper Working Example
- TL431 Battery Charger Voltage Detector Circuits Schematics
- TL431 Sink Mode Constant Current Circuits
- Physics and Chemistry for Electronics:
- Cesium Photo Detectors, Zinc Photocells, and Bandgap Explained
- Cesium Photoelectric Cells
- Is Zinc Photovoltaic?
- Bandgap versus Work Function Key Differences
- How Selenium Rectifiers and Photocells Operate
- Brief Overview of Vacuum Tubes and Circuits
- Basic Review Operation GaAs Photodiodes
- Electronics Education and Careers Defined Six Parts:
- Applied versus Theoretical Science Relation to Electronics
- How does applied science differ from theoretical science?
- Is electronics an applied science?
- What is the difference between electronics and electrical technicians?
- What does "academic ability" really mean in practical terms?
- How does an electronics technician differ from an engineer?
- Electronic Circuits and Applications:
- Electronics and Technology Built at Home Mainpage
- Arduino Constant Current H-Bridge Motor Control
- LM555 Timer Monostable AC Power Phase Control Demo